WATER QUALITY AND BIOLOGICAL CHARACTERISTICS OF EZEAGU WATERFALL
Journal: Water Conservation and Management (WCM)
Celestine Chukwuebuka Eneh, Ifeanyi Oscar Aguzie, Elijah Chibueze Odii, Nelson Jehosephat Nwankwo, Joseph Onyekwere Okoro, Ifeanyi Maxwell Ezenwa
Print ISSN : 2523-5664
Online ISSN : 2523-5672
This is an open access article distributed under the Creative Commons Attribution License CC BY 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Doi: 10.26480/wcm.04.2024.444.453
Abstract
 The water quality and biological characteristics of Ezeagu Waterfall was assessed during the second quarter of 2016. The study encompassed the analysis of physicochemical parameters as well as the composition and abundance of benthic macro-invertebrates. The physicochemical parameters: total hardness, pH, salinity, alkalinity, dissolved oxygen (DO), biological oxygen demand (BOD), chemical oxygen demand (COD), temperature, depth, chloride, sulphate and nitrate, were evaluated by standard procedures. Scoop net, serrated core sampler and Ekman grab served for sampling of macro-invertebrates. Indices of diversity, evenness and richness were used to compare biotic spatial composition of the waterfall. The pH for the duration of the study was acidic (3.83 ± 0.67 to 5.17 ± 0.12) and salinity ranged from 0.01 ± 0.00 ppt to 0.05 ± 0.04 ppt. Temperature variation was approximately 2ºC for the duration of the study. The highest total hardness was 18.00 ± 1.15 mg/L. The DO decreased significantly from April to June (p < 0.05). BOD and COD showed minimal month dependent changes. Concentration of chloride, nitrate and sulphate increased from April to June. A total of 73 macroinvertebrates in 11 orders, 17 families (including 4 unidentified) and 17 species were recovered. Neoperla sp., a stonefly was the most abundant species (23.3%), followed by Dystisecus marginalis (16.4%) and Caridina africana (13.7%). Simpson’s (D = 0.12629) and Gini-Simpson’s (1-D = 0.875371) indices indicated a high species diversity. Margalef (3.729204) and Menhinick (1.989700) indicated high species richness. Ezeagu waterfall was not polluted. This facilitated the thriving and proliferation of pollution-sensitive species, including Neoperla spp., Hydropsychid spp. and Phyllomacromia spp.
The water quality and biological characteristics of Ezeagu Waterfall was assessed during the second quarter of 2016. The study encompassed the analysis of physicochemical parameters as well as the composition and abundance of benthic macro-invertebrates. The physicochemical parameters: total hardness, pH, salinity, alkalinity, dissolved oxygen (DO), biological oxygen demand (BOD), chemical oxygen demand (COD), temperature, depth, chloride, sulphate and nitrate, were evaluated by standard procedures. Scoop net, serrated core sampler and Ekman grab served for sampling of macro-invertebrates. Indices of diversity, evenness and richness were used to compare biotic spatial composition of the waterfall. The pH for the duration of the study was acidic (3.83 ± 0.67 to 5.17 ± 0.12) and salinity ranged from 0.01 ± 0.00 ppt to 0.05 ± 0.04 ppt. Temperature variation was approximately 2ºC for the duration of the study. The highest total hardness was 18.00 ± 1.15 mg/L. The DO decreased significantly from April to June (p < 0.05). BOD and COD showed minimal month dependent changes. Concentration of chloride, nitrate and sulphate increased from April to June. A total of 73 macroinvertebrates in 11 orders, 17 families (including 4 unidentified) and 17 species were recovered. Neoperla sp., a stonefly was the most abundant species (23.3%), followed by Dystisecus marginalis (16.4%) and Caridina africana (13.7%). Simpson’s (D = 0.12629) and Gini-Simpson’s (1-D = 0.875371) indices indicated a high species diversity. Margalef (3.729204) and Menhinick (1.989700) indicated high species richness. Ezeagu waterfall was not polluted. This facilitated the thriving and proliferation of pollution-sensitive species, including Neoperla spp., Hydropsychid spp. and Phyllomacromia spp.Keywords
Ezeagu Waterfall, physicochemical, macroinvertebrates, abundance, diversity indices, water quality
1. INTRODUCTION
Water is the most abundant resource on planet Earth (Bwire et al., 2020). Clean, safe, and adequate water is vital for the survival of all living organisms and for the smooth functioning of ecosystems, communities, and economies (Matta et al., 2017). However, due to rising human population, industrialization, fertilizer use, and other anthropogenic activities, water is heavily contaminated with a variety of dangerous pollutants (Sharma et al., 2016). Water abstraction for domestic use, agricultural production, mining, industrial processes, power generation, and forestry practices can lead to deterioration in both water quality and quantity, impacting not only aquatic ecosystems but also the availability of safe water for human consumption (Programme, U. N. E. P. G. E. M. S., Gems/Water. 2008).
Water quality is defined in terms of its chemical, physical and biological contents (Makinde et al., 2015). The availability of good-quality water is an indispensable factor in preventing diseases and improving the quality of life (Sharma et al., 2016). To comprehend the extent and nature of contamination, continuous monitoring of water quality is essential. This necessitates a robust monitoring system that encompasses the physical, chemical, and biological components of freshwater ecosystems (Magbanua et al., 2023). Understanding physicochemical factors can provide insights into the productivity of a water resource, guide the choice of appropriate water treatment procedures, and assess the potential for thriving populations of species. Recognizing the significance of physicochemical parameters in water, the World Health Organization (WHO) recommends regular monitoring to ensure they remain within acceptable limits (WHO. 2008). Depending on the level of contamination, appropriate curative or preventive measures must be implemented to restore water quality (Renu Nayar, 2020).
Biological monitoring, often referred to as biomonitoring, involves systematically utilizing living organisms or their responses to assess the quality of the aquatic environment (Barbour et al., 1999). The use of sentinel species (bio-indicators) has been traditionally used in studies of bio-monitoring, including environmental risk assessment (Friberg et al., 2011). The underlying principle of using bio-indicator species for water quality assessment is based on the notion that the presence of these organisms reflects the overall environmental health (Johnson and Wiederholm, 1993).
Surface waters, including perennially flowing streams, are heavily stressed due to their diverse uses for water supply, agriculture, industry, and recreation. This extensive use renders these waters susceptible to contamination (Walkeret al., 2019). Ensuring safe and reliable water for global populations while promoting the sustainable utilization of water resources stands as a fundamental objective of the Millennium Sustainable Goals. As a consequence, water sources worldwide undergo periodic analysis. Waterfalls, most of which originate from streams or rivers cascading from high elevations over cliffs or rocks, have received minimal attention from researchers worldwide (Offem et al., 2012). The remote location of Ezeagu Waterfall in Enugu State, Nigeria, has hindered comprehensive analytical investigations in that area. The present study seeks to determine the physicochemical and biological characteristics of this water body.
2. MATERIALS AND METHODS
2.1 Study Area and Sampling Stations
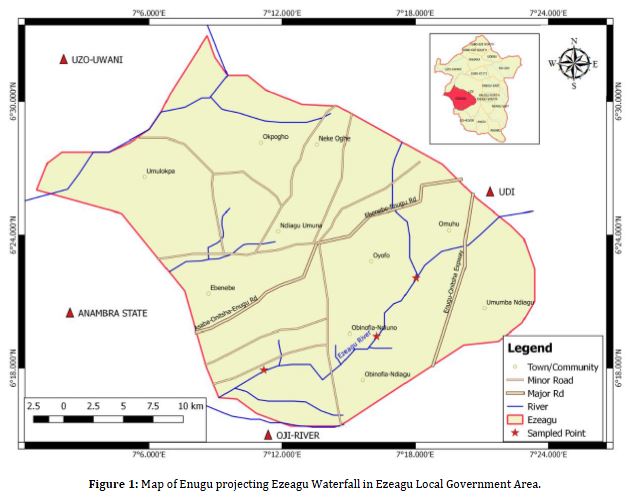
Ezeagu Waterfall, also known as Ezeagu River, is locally referred to as Agada or Okpaku by the Umuagu community. It is situated in Omughu Obeleagu Umana, within the Ezeagu Local Government Area of Enugu State, Nigeria. Geographically, the waterfall lies between latitude 6°25’N and longitude 7°15’E (Figure 1).
Enugu State is located in the southeastern part of Nigeria, sharing its borders with Abia and Imo States to the south, Ebonyi State to the east, Benue State to the northeast, Kogi State to the northwest, and Anambra State to the west. The state benefits from a favorable year-round climate and soil conditions, positioned at an elevation of approximately 223 meters (73 ft.) above sea level. The soil is well-drained during the rainy seasons. The hottest month, February, records an average temperature of 30.64°C (87.16°F), while the coolest temperatures occur in November, dipping to 15.86°C (60.54°F). The lowest rainfall, around 0.16 cubic centimeters (0.0098 cu in.), is typical in February, contrasting with the highest, 35.7 cubic centimeters (2.18 cu in.), observed in July (Okeibunor et al., 2013).
Ezeagu Waterfall is a spring that spans approximately 126 meters in width, featuring varying depths ranging from 0.8 to 3.2 meters. It descends from a 23-meter-high cliff. This stream significantly contributes to the water supply of the Umuagu community, also serving as a tourist attraction and supporting agricultural and domestic uses.
Three sampling sites, termed as Stations 1, 2, and 3, were chosen along the length of the waterfall, approximately 30 meters apart. These stations were selected based on their distinct features. Station 1 was located upstream, Station 3 downstream, and Station 2 in the middle of the waterfall, benefiting from direct sunlight penetration.
2.2 Collection, Processing, and Characterization of Water and Macroinvertebrate Samples
Sampling was designed to include the early to peak periods of the rainy season. For three months (April to June), water samples and sediments were collected monthly at each sampling site. A 150 ml plastic container was used for collection. Prior to use, the containers underwent thorough cleaning with 5% nitric acid, followed by rinsing with distilled water, and drying to eliminate any potential impurities. This procedure adheres to the methods outlined by (Wangboje and Oronsaye, 2001). For sediment collection, an Eckman grab sampler was employed, and the collected sediments were then carefully placed into appropriately labeled plastic bags. Once collected, both water and sediment samples were transported to the laboratory within a 24-hour window and stored at a temperature of 5°C before analysis.
Water temperature was determined in situ using clinical mercury in glass thermometer. The depth of water at each sampling station was measured according to (Warner and Hughes, 1998). The monthly hydrogen ion concentration (pH) was determined in the field with the use of a digital pH meter (model EIL 3055). Dissolved oxygen (DO) was determined using the Winkler’s method (Boyd et al., 1979). The biochemical oxygen demand (BOD) was determined using the permanganate method (Chapman, 2021). Alkalinity and salinity were determined using the titration method. Water hardness was determined in the laboratory using Erichrome Black T indicator method. The nitrate (NO3-), sulphate (SO4) and chloride (Cl) were determined using the ultraviolet spectrophotometric and Mohr’s method, respectively (APHA. 2012). Chemical oxygen demand (COD) was determined by titrimetric method
Macroinvertebrates were collected utilizing a 0.05 μm mesh size scoop net, a serrated core sampler, and an Ekman grab. The kick-sweep method was also employed during the sampling process. This technique involves kicking the riverbed for three minutes, which causes organisms to be dislodged and trapped. Larger stones within the sampled area were gently rubbed to dislodge clinging organisms, enabling them to be swept into the net. Quantitative sampling was carried out using a serrated core sampler. The mesh net containing the collected samples was then inverted and gently shaken within a plastic container filled with water, helping to separate leaves, rocks, and other debris from the collected organisms. The serrated core sampler was emptied out into containers for sorting. Sorting was done in the laboratory. The macroinvertebrates were preserved in a 70% ethyl alcohol. Identification was by means of a dichotomous key by (Umar et al., 2015).
3. DATA ANALYSIS
Data was analyzed using Statistical Package for Social Sciences (SPSS) version 20 (IBM Corp., Amonk, New York) and Microsoft Office Excel (Microsoft Inc., Redmond, USA). Two-way analysis of variance (ANOVA) was used to compare physicochemical parameters between the stations and the months. Percentage abundance, diversity, evenness, and richness indices were calculated. Simpson’s index, Gini-Simpson, Reciprocal Simpson, Shannon-Wiener diversity index, Modified Shannon-Wienner index, Berger-Parker index, McIntosh index, Margalef index, Menhinick index, Hill’s family of numbers (N0, N1 and N2), Sheldon’s index, Heip index, Pielo’s index and Simpson’s index of evenness were all calculated according to the formulae listed by (Ludwig and Reynolds,1988; Krebs, 2014). P ≤ 0.05 was considered as significant
4. RESULTS
4.1 Physicochemical Characteristics of Ezeagu Waterfall
The overall physicochemical characteristic of Ezeagu Waterfall at the three sampled locations during the study is presented in Table 1.

Variation observed in the physicochemical characteristics of Ezeagu Waterfall during the study was dependent on the sampled station, and the month samples were collected. Significant variations were observed between the stations for some of the parameters, while some were virtually unchanged. Significant variations from month to month were also observed for some of the parameters. There was approximately 2 ºC variation in the temperature of the water for the duration of the study. The peak temperature observed for the duration of the study was 29ºC in May and the least was 26.67 ºC (± 0.33) in June. Temperature decreased in stations 1 and 3 between April and June. The depth of the water at the sampling points ranged from 0.70 ± 0.18 m to 1.35 ± 0.26 m. Water depth varied significantly in April (p < 0.05). Salinity of Ezeagu Waterfall ranged from 0.01 ppt in May to 0.05 ± 0.04 ppt in June. Salinity of Ezeagu Waterfall only changed slightly between April and June (Table 2).

At all the stations pH for the duration of the study was acidic (3.73 ± 0.67 to 5.17 ± 0.12). pH between the three sampled stations only differed significantly in April (p < 0.05). The pH at stations 2 and 3 decreased significantly in June compared to April and May. At station 2, the pH decreased from 4.90 ± 0.58 in April and 4.87 ± 0.03 in May to 3.73 ± 0.67 in June. Similarly, at station 3, the pH decreased from 4.70 ± 0.57 in April and 5.17 ± 0.12 in May to 3.83 ± 0.67 in June. The alkalinity of Ezeagu Waterfall ranged from a maximum of 46.18 ± 1.18 mgL-1 at station 3 to a minimum of 33.13 ± 1.12 mgL-1 at station 2. The alkalinity of the water body decreased from April to June. The decrease was significant between April and June at all the stations (p < 0.05). Alkalinity of Ezeagu Waterfall was never significantly different between the stations for the months of the study. Ezeagu Waterfall generally had low total hardness values. The highest total hardness value was 18.00 ± 1.15 mgL-1. At station 1, total hardness of the water increased from 10.00 ± 2.31 in April to 18.00 ± 1.15 mgL-1 in May, and decreased to 6.00 ± 1.15 mgL-1 in June (p < 0.05). The Biological Oxygen Demand (BOD) of the water was between 3.28 ± 0.06 mgL-1 and 4.03 ± 0.19 mgL-1 for the duration of the study. No significant variation occurred in the BOD between the stations. The COD of Ezeagu Waterfall ranged from 23.70 ± 0.58 mgL-1 to 26.12 ± 0.15 mgL-1 for the duration of the study. In the month of April, the COD of station 3 was significantly higher than station 1 and station 2 values. With the exception of station 3, there were no significant variations in the values of COD between any two months. In station 3, the COD decreased significantly from 26.12 ± 0.15 mgL-1 in April to 23.59 ± 0.42 mgL-1 in May. The Dissolved oxygen (DO) in Ezeagu Waterfall for the duration of the study ranged from 4.83 ± 0.19 to 7.37 ± 0.30. The values of DO decreased significantly from April to June in stations 1 (6.61 ± 0.06 to 4.97 ± 0.48), station 2 (6.70 ± 0.46 to 4.83 ± 0.19) and station 3 (7.37 ± 0.30 to 4.90 ± 0.15). No significant difference was observed in the DO values between any two stations (Table 3).
4.2 Variations in the Nutrient Composition of Ezeagu Waterfall
The maximum and minimum concentration of chloride in Ezeagu Waterfall was 23.31 ± 1.38 mgL-1 and 21.80 ± 0.60 mgL-1 respectively. Chloride concentration only increased progressively from April (22.19 ± 0.44 mgL-1) through May (22.77 ± 0.62 mgL-1) to June (23.93 ± 0.18 mgL-1) in station 3. The difference between chloride concentration in April and June for station 3 was significant (p < 0.05). Difference in the concentration of chloride between the stations was noticed only in June where the chloride concentration of station 3 was significantly higher than that of station 2 (p < 0.05). The concentration of sulphate at the stations ranged between 0.12 ± 0.00 mgL-1 to 0.74 ± 0.02 mgL-1. Sulphate concentration increased significantly from April through May to June in all the stations. Significant difference in sulphate concentrations between the stations was observed only in June: the concentration of sulphate in station 3 was significantly higher than other stations. The nitrate concentrations at the three stations were never significantly different from each other for the three months of the study. Though at station 2 significant difference was observed between April and June nitrate concentrations where the level increased from April to June (Table 4).



The multi-dimensional physicochemical relationships necessitated a principal component analysis (PCA). PCA reduced the dimensions into four principal components (PCs) which cumulatively explained76.9% of total variations in physicochemical characteristics of the waterfall in the period studied. In the first PC, which explained 38.7% of variance, alkalinity, sulphate, and DO loaded strongly (r ≥ 0.75), while temperature and pH loaded moderately (0.75 ≥ r ≥ 0.50). Two variables loaded strongly in the second PC, total hardness and salinity, while pH and nitrate loaded moderately. In the third and fourth PCs, COD and chloride loaded strongly respectively (Table 5).

4.3 Macroinvertebrates Species and Abundance in Ezeagu Waterfall
A total of 73 macro-invertebrates in 11 orders (Coleopterans, Decapoda, Hemiptera, Odanata, Plecoptera, Trichoptera, Ephemeroptera, Araneae, Isoptera, Blatodea and Magadrilacea), 17 families including 4 unidentified (Dysticidae, Hydraenidae, Aeshinidae, Nepidae, Corixidae, Atyidae, Astacidae, Libellulidae, Macromiidae, Perlidae, Baetidae, Blaberidae and Hydropsychidae); and 17 species were collected from the waterfall. The species include Astacopsis sp., Dystiscus marginalis, Caridina africana, Ranatra linearis, Corixa punctuata and Acisoma sp.
(Table 6, Figure 3).

The most represented Order was Coleoptera with 3 families; while Dysticidae was the family with the highest numbers of species representation at Ezeagu Waterfall. The species with the highest abundance was Neoperla sp. (23.3%) followed by D. marginalis (16.4%) and Caridina africana (13.7%). The least abundant species were R. linearis, Phyllomacromia sp., and the unidentified species under Hydraenidae, Magadrilacea, Isoptera and Blatodae (1.4%). In station 1, the most abundant species were D. marginalis (18.2%), Astacopsis sp. (18.2%) and Neoperla sp. (18.2%). In station 2 there was almost a uniform abundance of species; out of the 8 species found in that location, 7 had 11.1% abundance, and only D. marginalis had 22.2% abundance. Neoperla sp. was the most abundant species (28.6%) in station 3.


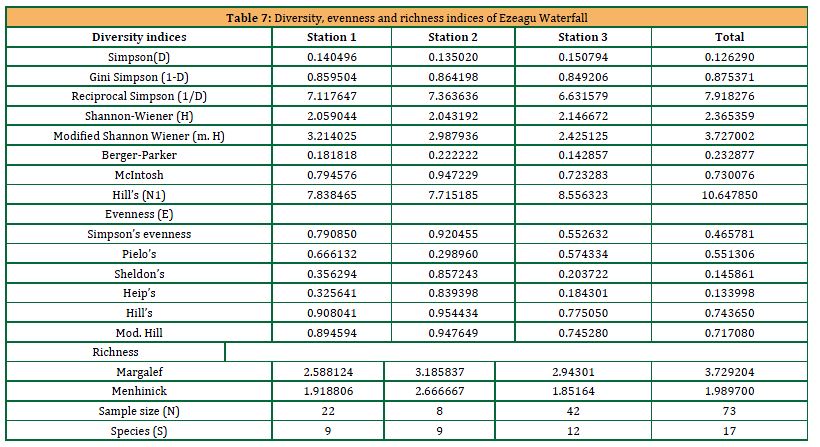
4.4 Diversity, Evenness and Richness Indices of Ezeagu Waterfall
The Simpson’s and Gini-Simpson’s indices for station 1 (0.140496 and 0.859504), station 2 (0.13502 and 0.864198), station 3 (0.150794 and 0.849206) and overall (0.12629 and 0.875371) indicates a high species abundance (Table 7). From the reciprocal Simpson’s index (equivalent to Hill’s N2), there were approximately 7 very highly abundant species in station 1 (N2 = 7.117647), 7 very highly abundant species in station 2 (N2 = 7.363636), 7 very highly abundant species in stations 3 (N2 = 6.631579) and 8 very highly abundant species overall in Ezeagu Waterfall (N2 = 7.918276). There were eight highly abundant species according to Hill’s N1 in station 1 (N1 = 7.838465), eight in station 2 (N1 = 7.715185), nine in station 3 (N1 = 8.556323), and eleven overall in Ezeagu Waterfall (N1 = 10.64785). Station 2 from Simpson’s (E = 0.920455), Sheldon’s (E = 0.857243), Hill’s (E = 0.954434) and Heip’s (E = 0.839398) indices had the highest species evenness. Margalef and Menhinick’s indices ascribed respectively 2.588124 and 1.918806 to station 1; 3.185837 and 2.66667 to station 2, and 2.94301 and 1.85164 to station 3. Thus, the species richness of the stations according to these indices was in the order: Station 2 > Station 3 > Station 1.
5. DISCUSSION
The physicochemical parameters of Ezeagu Waterfall displayed fluctuations in the levels of both physical and chemical attributes of the water between April and June. These fluctuations were characterized by both increases and decreases. The water quality parameters, temperature have considerable impacts on the aquatic ecosystem species (Meshesha et al., 2020). Minimum and maximum temperatures of 25.00°C and 35.50°C, respectively are typical of tropical waters and are essential for the proper growth of aquatic organisms (Oboh and Agbala, 2017). In the case of Ezeagu Waterfall, the temperature exhibited a consistent decrease from April to June (28.67°C ± 0.33 to 26.67°C ± 0.33). This temperature variation is likely attributed to fluctuations in solar radiation intensity, coupled with increased water volume and current due to the transition from the late dry/early rainy season in April to the fully rainy season in June. The mean water temperature observed during the study period remained within the standard permissible limits set by (Beszczynska-Möller et al., 2012). Comparable temperature observations have been documented for rivers in Nigeria as well as in other regions (Atobatele and Ugwumba, 2008; Adesakin et al., 2020; Vijayakumar et al., 2014).
The pH levels observed at the sampled stations exhibited a shift towards acidity from April to June (ranging from 3.73 ± 0.67 to 5.17 ± 0.12). This trend coincided with the transition from the dry to the rainy season. The pH of water depend on the geology and soils of the area (Numbere, 2017). The increased acidity can be attributed to the influx of organic matter carried by rainfall during the peak wet season, resulting in runoff. A decrease in dissolved oxygen levels and a dip in pH are the end results of this runoff’s contribution to the utilization of organic material through dehydration. However, the pH values determined in this study fell outside the range of surface water quality standards outlined by previous literature (Saalidong et al., 2022). This observation is consistent with the pH values reported in similar research conducted in Opi Lake (Onah et al., 2022). Moreover, it supports the assertion from the (UNEP GEMS/Water Programme, 2008) that rainfall naturally introduces acidity due to the dissolution of CO2. The substantial presence of dense vegetation in the vicinity may have contributed to the accumulation of organic materials that subsequently decomposed, further contributing to the observed acidic pH. Additionally, the underlying composition of the water body’s base, whether stony or sandy, could account for variations in buffering capacity. As a result, station 1, with a stony base, exhibited higher pH levels, while the sandy nature of station 3 might have contributed to the observed lower pH levels.
The levels of both BOD and COD remained relatively consistent across all sampled stations throughout the study duration. The biochemical oxygen demand was very low during this study. Specifically, BOD values below 6 mgL-1 indicate a lower presence of organic pollutants and suggest a water environment conducive to supporting aquatic life (Oluyemi et al., 2011). The BOD values observed at the waterfall fell within the recommended range for surface water quality. Hence, Ezeagu waterfall is therefore suitable for drinking. The water’s hardness gives an indicator of its capacity to withstand high soap concentration. The concentration of the total hardness is far lower than the permissible level of 150mg/l. Similarly, low values of water hardness were recorded in a study conducted in Sagbama Creek Niger Delta Nigeria (Seiyaboh et al., 2017).
The levels of dissolved oxygen (DO) were consistently low. Notably, these levels were even lower at the peak of the rainy season in June, which contradicts findings from other researcher (Ryan et al., 2020), who reported higher dissolved oxygen during the rainy season. Similar results were documented in previous works (Adedeji et al., 2019; Adedayo, 2016), demonstrating comparable dissolved oxygen values. This decrease in dissolved oxygen could be attributed to excessive algae and phytoplankton growth driven by high levels of phosphorus and nitrogen (Woldeab et al., 2023). This decomposition can lead to an increase in the composition of algae and other microorganisms in the water, which subsequently contributes to oxygen depletion (Programme, U. N. E. P. G. E. M. S., Gems/Water. 2008). Moreover, the level of pH in the water body influences dissolved oxygen levels, impacting both respiration and photosynthesis processes. Despite these fluctuations, the recorded ranges of dissolved oxygen remained within the minimum recommended values.
Nutrient levels varied noticeably over the course of the study. Nitrate is an essential nutrient for the growth of phytoplankton. Remarkably, the levels of nitrate were consistently low. This pattern of low nitrate values aligns with findings from other researchers (Woldeab et al., 2023), who recorded similarly low values. They reported the lowest nitrate concentration of 0.26 ± 0.015 mgL-1 in the rainy month of July and the highest value of 4.15 ± 0.127 mgL-1 in the dry month of February. The minimal variation in nitrate concentration observed in our study could potentially be attributed to different hydrogeological regimes. In June, downstream at station 3 exhibited higher chloride and sulfate concentrations. Chloride levels progressively increased from April to June at station 3, while sulfate showed consistent increases across all stations. High concentration of chloride from this study could be due to uses of chlorine as a disinfectant in water purification (Adesakin et al., 2020). Importantly, the recorded chloride ranges from surface water samples fell within the stipulated limits set by the WHO for potable water quality. Nutrient content in rivers is influenced by factors like flow intensity, changing sources, and water conditions. This study revealed a low mean sulphate values from surface water sources and were within the WHO and SON stipulated limits of 250 mgL-1. The low concentration of sulphate could be due to the absence of anthropogenic activities that influence the concentration in water bodies (Adesakin et al., 2020). The variability in sulfate levels may stem from multiple sources, including decomposition of organic matter, rain-induced organic material inflow, and shifts in water characteristics. The reasons for chloride fluctuations, however, appear less distinct compared to sulfate.
Macroinvertebrates serve as crucial indicators in ecological assessments of aquatic ecosystems since the composition and richness of their communities provide insights into environmental and anthropogenic changes (Brantschen et al., 2022). Most of the macroinvertebrates identified in this study are found throughout Nigeria (Onah et al., 2022; Arimoro and Keke, 2017; Arimoro et al., 2015). The moderate species diversity in the river can be attributed to specific physicochemical conditions, such as low pH and low dissolved oxygen (DO). Additionally, the moderate abundance (number of individuals) and diversity of benthic invertebrates documented in this study could be linked to the heterogeneous nature of the vegetation within the littoral zone of the study stations. This diverse vegetation likely provided a suitable habitat for a wide range of benthic fauna (Arimoro and Keke, 2017). Aquatic insects were the predominant benthic macroinvertebrates in the Ezeagu Waterfall. Regarding the taxonomic distribution of macro-invertebrates, Plecopterans were the most dominant, followed by Decapods and Coleopterans. This differs from findings in previous research works who reported Odonata, Trichoptera, and Diptera as the most abundant in southeastern and southern Nigerian water bodies (Adedeji et al., 2019; Ezenwa et al., 2023; Olomukoro, and Ezemonye, 2007). Plecopterans have a reputation for living in clean, well-oxygenated, little-polluted environments at fairly cool temperatures (Saal et al., 2021). Also, the abundance of Coleoptera in most of the stations is an indication that these sites are relatively free from gross pollution (Arimoro and Keke, 2017). Neoperla species stood out as the dominant species occurring in all three stations. Stoneflies like Neoperla are reliable indicators of water pollution levels due to their sensitivity to oxygen content (Myers et al., 2011). This is because their gills are located along the body, effectuating this family’s dependence on high dissolved oxygen in the water to respire (Ab Hamid and Md Rawi, 2017). Their absence in highly polluted, oxygen-depleted waters suggests that Ezeagu Waterfall was unpolluted during the study. A few species from the Odonata and Ephemeroptera orders, indicative of clean water quality, were also present. The presence of Coleoptera in an aquatic system, along with other less tolerant species such as Ephemeroptera, Plecoptera, Tricoptera, and Odonata has been observed to reflect clean water conditions (Miserendino and Pizzolon, 2003).
6. CONCLUSION
The macro-invertebrate diversity of Ezeagu Waterfall and its physicochemical characteristics are indicative of clean water or, reservedly, minutely contaminated water. Therefore, the water is favorable to macroinvertebrates. Its high acidity, however, may reduce its usefulness to human.
ACKNOWLEDGMENTS
The authors wish to thank the Department of Biochemistry and Zoology & Environmental Biology, University of Nigeria, Nsukka, Enugu State, for their assistance with laboratory resources throughout the course of this research.
FUNDING
The authors declare no specific funding for this work
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Ab Hamid, S., and Md Rawi, C. S., 2017. Application of Aquatic Insects (Ephemeroptera, Plecoptera and Trichoptera) in Water Quality Assessment of Malaysian Headwater. Tropical Life Sciences Research, 28(2), Pp. 143–162. https://doi.org/ 10.21315/ tlsr2017.28.2.11
Adedayo, E., 2016. Physicochemical Analysis of the Coastal Waters of Ondo State ,. International Journal of Research in Agriculture and Forestry, 3(11), Pp. 13–20.
Adedeji, H., Idowu, T. A., Usman, M. A., and Sogbesan, O., 2019. Seasonal variations in the physico-chemical parameters of Lake Ribadu, Adamawa state Nigeria. International Journal of Fisheries and Aquatic Studies, 7(1), Pp. 79–82. https://doi.org/10.13140/RG.2.2.19472.87044
Adesakin, T. A., Oyewale, A. T., Bayero, U., Mohammed, A. N., Aduwo, I. A., Ahmed, P. Z., Abubakar, N. D., and Barje, I. B., 2020. Assessment of bacteriological quality and physico-chemical parameters of domestic water sources in Samaru community, Zaria, Northwest Nigeria. Heliyon, 6(8), e04773. https://doi.org/10.1016/j.heliyon.2020.e04773
APHA, 2012. American Public Health Association. Standard Methods for examination of water and wastewater. ISBN 978-087553-013-0
Arimoro, F. O., and Keke, U. N., 2017. The intensity of human-induced impacts on the distribution and diversity of macroinvertebrates and water quality of Gbako River, North Central, Nigeria. Energy, Ecology and Environment, 2(2), Pp. 143–154. https://doi.org/10.1007/s40974-016-0025-8
Arimoro, F. O., Odume, O. N., Uhunoma, S. I., and Edegbene, A. O., 2015. Anthropogenic impact on water chemistry and benthic macroinvertebrate associated changes in a southern Nigeria stream. Environmental Monitoring and Assessment, 187(2), Pp. 14. https://doi.org/10.1007/s10661-014-4251-2
Atobatele, O. E., and Ugwumba, O. A., 2008. Seasonal variation in the physicochemistry of a small tropical reservoir (Aiba Reservoir, Iwo, Osun, Nigeria). African Journal of Biotechnology, 7(12), Pp. 1962–1971. https://doi.org/10.5897/AJB2008.000-5043
Barbour, M.T., Gerritsen, J., Snyder, B.D. and Stribling, J. B., 1999. Rapid Bioassessment Protocols for Use in Stream and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish (2nd Editio). U.S. Environmental Protection Agency, Office of Wate.
Beszczynska-Möller, A., Fahrbach, E., Schauer, U., and Hansen, E., 2012. Variability in Atlantic water temperature and transport at the entrance to the Arctic Ocean, 1997–2010. ICES Journal of Marine Science, 69(5), Pp. 852–863.https://doi.org/10. 1093/icesjms/fss056
Boyd, C. E., Romaire, R. P., and Johnston, E., 1979. Water Quality in Channel Catfish Production Ponds. Journal of Environmental Quality, 8(3), Pp. 423–429. https://doi.org/10.2134/jeq1979.00472425000800030031x
Brantschen, J., Pellissier, L., Walser, J., and Altermatt, F., 2022. Evaluation of primer pairs for eDNA‐based assessment of Ephemeroptera, Plecoptera, and Trichoptera across a biogeographically diverse region. Environmental DNA, 4(6), Pp. 1356–1368. https://doi.org/10.1002/edn3.342
Bwire, G., Sack, D. A., Kagirita, A., Obala, T., Debes, A. K., Ram, M., Komakech, H., George, C. M., and Orach, C. G., 2020. The quality of drinking and domestic water from the surface water sources (lakes, rivers, irrigation canals and ponds) and springs in cholera prone communities of Uganda: an analysis of vital physicochemical parameters. BMC Public Health, 20(1), 1128. https://doi.org/10.1186/s12889-020-09186-3
Chapman, D., 2021. Water Quality Assessments. CRC Press. https://doi.org/10.1201/978 1003062103
Ezenwa, I. M., Ekechukwu, N., Ukwueze, C., Okafor, G., Orakwelu, C. H., Ezeorah, C. C., Hinmikaiye, F. F., Ngene, C. I., Omoigberale, M., and Nwani, C., 2023. Anthropogenic induced physicochemical gradients and associated macroinvertebrate community changes in derived savannah stream in Nigeria: Implication for biotic assessment. Acta Ecologica Sinica, 43(3), Pp. 535–544. https://doi.org/10.1016/ j.chnaes.2022.06.003
Friberg, N., Bonada, N., Bradley, D. C., Dunbar, M. J., Edwards, F. K., Grey, J., Hayes, R. B., Hildrew, A. G., Lamouroux, N., Trimmer, M., and Woodward, G., 2011. Biomonitoring of Human Impacts in Freshwater Ecosystems. Pp. 1–68. https://doi.org/10.1016/B978-0-12-374794-5.00001-8.
Johnson R. K., Wiederholm T., R. D. M., 1993. Freshwater Biomonitoring Using Individual Organisms, Populations and Species Assemblages of Benthic Macroinvertebrates. Chapman and Hall.
Krebs, C. J., 2014. Ecological methodology (Third). Pearson PLC.
Ludwig, J. A., and Reynolds, J. F., 1988. Statistical ecology: a primer in methods and computing. John Wiley and Sons, Ltd.
Magbanua, F. S., Hilario, J. E., Salluta, J. C. R. B., Alpecho, B. C., Mendoza, S. S., and Lit, I. L., 2023. Freshwater biomonitoring with macroinvertebrates in the Philippines: Towards the development of the Philippine biotic index. Limnologica, 102, 126098. https://doi.org /10.1016/j.limno.2023.126098
Makinde, O. O., Edun, O. M., and Akinrotimi, O. A., 2015. Comparative Assessment of Physical and Chemical characteristics of Water in Ekerekana and Buguma Creeks , Niger Delta Nigeria. Journal of Environment Protection and Sustainable Development, 1(3), Pp. 126–133.
Matta, G., Srivastava, S., Pandey, R. R., and Saini, K. K., 2017. Assessment of physicochemical characteristics of Ganga Canal water quality in Uttarakhand. Environment, Development and Sustainability, 19(2), Pp. 419–431. https:// doi.org/ 10.1007/s10668-015-9735-x
Meshesha, T. W., Wang, J., and Melaku, N. D., 2020. Modelling spatiotemporal patterns of water quality and its impacts on aquatic ecosystem in the cold climate region of Alberta, Canada. Journal of Hydrology, 587, 124952.https://doi.org/10.1016/j. jhydrol. 2020.124952
Miserendino, M. L., and Pizzolon, L. A., 2003. Distribution of macroinvertebrate assemblages in the Azul‐Quemquemtreu river basin, Patagonia, Argentina. New Zealand Journal of Marine and Freshwater Research, 37(3), Pp. 525–539. https://doi.org/10.1080 00288330.2003.9517187
Myers, L. W., Kondratieff, B. C., Mihuc, T. B., and Ruiter, D. E., 2011. The Mayflies (Ephemeroptera), Stoneflies (Plecoptera), and Caddisflies (Trichoptera) of the Adirondack Park (New York State). Transactions of the American Entomological Society, 137(1 & amp; 2), Pp. 63–140. https://doi.org/10.3157/061.137.0118
Vijayakumar, N., Shanmugavel, G., Sakthivel, D., and Anandan, V., 2014. Seasonal variations in physico-chemical characteristics of Thengaithittu estuary, Puducherry, South East-Coast of India. Advances in Applied Science Research, 5(5), Pp. 39–49. www.pelagiaresearchlibrary.com
Numbere, A. O., 2017. Concentrations of Iron and Other Physico-Chemical Parameters in Ground and Surface Water in Some Mangrove Forest Areas in the Niger Delta, Nigeria. African Journal of Applied Zoology and Environmental Biology. 2017, 19(April), Pp. 1–12. www.uniportjournals.com/ajazebajazeb_ng@yahoo.com
Oboh, I., and Agbala, C., 2017. Water quality assessment of the Siluko River, southern Nigeria. African Journal of Aquatic Science, 42(3), Pp. 279–286. https://doi.org/10.2989/160859 14.2017.1371579
Offem, B. O., and Ikpi, G. U., 2012. Distribution and dynamics of a tropical waterfalls ecosystem. Knowledge and Management of Aquatic Ecosystems, 404, 10. https://doi. org/10.1051/kmae/2012004
Okeibunor, J. C., Onyeneho, N. G., Nwaorgu, O. C., I’Aronu, N., Okoye, I., Iremeka, F. U., and Sommerfeld, J., 2013. Prospects of using community directed intervention strategy in delivering health services among Fulani Nomads in Enugu State, Nigeria. International Journal for Equity in Health, 12(1), Pp. 1–17. https://doi.org/10.1186/ 1475-9276-12-24
Olomukoro, J. O., and Ezemonye, L. I. N., 2007. Assessment of the macro-invertebrate fauna of rivers in southern Nigeria. African Zoology, 42(1), Pp. 1–11. https://doi.org/ 10.1080/15627 020.2007.11407371
Oluyemi, E. A., Adekunle, A. S., Adenuga, A. A., and Makinde, W. O., 2011. Physico-chemical properties and heavy metal content of water sources in Ife North Local Government Area of Osun State, Nigeria. African Journal of Environmental Science and Technology, 4(10), Pp. 156–158. http://www.academicjournals.org/AJEST
Onah, I., Ajanwachukwu, O., and Ubachukwu, P., 2022. Comparison of physico-chemical parameters with macroinvertebrate and vertebrate fauna of Lake Ogelube and Lake Ojii, Opi-Agu, south-eastern Nigeria. African Journal of Aquatic Science, 47(4), Pp. 489–498. https://doi.org/10.2989/16085914.2022.2080174
Programme, U. N. E. P. G. E. M. S., Gems/Water. 2008. Water quality for ecosystem and human health (2nd editio). Ontario, Canada: UNEP Gems/Water Programme.
Renu Nayar. 2020. Assessment of Water Quality Index and Monitoring of Pollutants by Physico-Chemical Analysis in Water Bodies: A Review. International Journal of Engineering Research And, V9(01), Pp. 178–185. https://doi.org/10.17577/ ijertv9is010046.
Ryan, M. U., Bookout, B., and Kaushal, S. S., 2020. Influence of temperature, precipitation, and cloud cover on diel dissolved oxygen ranges among headwater streams with variable watershed size and land use attributes. Aquatic Sciences, 82, Pp. 82. https://doi.org/10.1007/s00027-020-00756-6
Saal, I., Bouchelouche, D., Hamache, C., and Arab, A., 2021. Evaluation of the surface water quality in the Kebir-Rhumel catchment area (northeast Algeria) using biotic indices and physico-chemical analyses. Environmental Science and Pollution Research, 28(34), Pp. 46565–46579. https://doi.org/10.1007/s11356-020-10598-2
Saalidong, B. M., Aram, S. A., Otu, S., and Lartey, P. O., 2022. Examining the dynamics of the relationship between water pH and other water quality parameters in ground and surface water systems. Published: January 25, 2022. https://doi.org/10.1371/ journal.pone.0262117
Seiyaboh, E. I., Izah, S. C., and Oweibi, S., 2017. Assessment of Water quality from Sagbama Creek, Niger Delta, Nigeria. Biotechnological Research, 3(1), Pp. 20–24.
Sharma, V., Kumar, Y., and Kumar, A., 2016. Assessment of Physico Chemical Parameters for Analysing Water : A Review Assessment of Physico Chemical Parameters for Analysing Water : A Review. J. Biol. Chem. Chron., 2(1), Pp. 25–33.
Umar, D. M., Harding, J. S., and Winterbourn, M. J., 2015. Freshwater Invertebrates of the Mambilla Plateau, Nigeria.
Walker, D. A., Epstein, H. E., Sibik, J., Bhatt, U., Romanovsky, V. E., Breen, A. L., Chasnikova, S., Daanen, R., Druckenmiller, L. A., Ermokhina, K., Forbes, B. C., Frost, G. V., Geml, J., Kaarlejärvi, E., Khitun, O., et al., 2019. Vegetation on mesic loamy and sandy soils along a 1700-km maritime Eurasia Arctic Transect. Applied Vegetation Science, 22(1), Pp. 150–167. DOI:10.1111/avsc.12401
Wangboje, O. M., and Oronsaye, J. A. O., 2001. Bioaccumulation of iron, lead, mercury, copper, zinc and chromium by fish species from Ogba River, Benin City, Nigeria. African Journal of Zoology and Environmetal Management, 3, Pp. 45 – 49.
Warner R. R., H. T. P., 1998. The population dynamics of Cichlid fishes. Proceeding of 6th International Coral Reef Symposium, 1: Pp. 149-155.
WHO. 2008. Guidelines for Drinking-water Quality: Second addendum. World Health Organization Press, 1, Pp.17–19. http://www.who.int/water_sanitation_health/dwq/ seconddad dendum20081119.pdf
Woldeab, B., Ambelu, A., Efrem, Z., Deribe, S., Megersa, M., Alemu, T., and Mereta, S. T., 2023. Depth profile of reservoir water quality in the Southwest of Ethiopia. Heliyon, 9(7), e17474. https://doi.org/10.1016/j.heliyon.2023.e17474
| Pages | 444-453 |
| Year | 2024 |
| Issue | 4 |
| Volume | 8 |

